TP9: Mechanosensitive and astrocytic contributions to synaptic plasticity during aging (Daniela C. Dieterich)
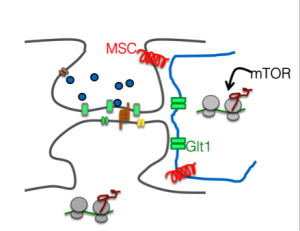
 Aging is a multifactorial process at the synaptic site, with alterations in protein expression levels, receptor abundances and deficits in synaptic potentiation. Astrocytes appear to play a pivotal role in these processes, as indicated by observed changes their supply of signaling molecules and cholesterol, as well as altered expression of proteins involved in neuron-glia communication (Perea et al., Mauch et al). Moreover, astrocytic secretion of ECM molecules critically contributes to the mechanoelastic properties of circuits in the brain and its change contributes to the increase rigidity of the extracellular environment increases in the aging brain. Such changes in of rigidity or viscoelasticity is sensed by neuronal mechanoreceptors and directly impacts on neuronal morphology and gene expression (Franze, 2013). However, the molecular mechanism of astrocytic aging and their effect on the ECM are poorly understood. To address the consequences of observed age-dependent changes in viscoelasticity, we recently adopted polyacrylamide (PAA)-based hydrogels to mimic these age-dependent changes in stiffness from young (soft, compliant, 0.1 kPa) to adult (1 kPa) and to very stiff (10 kPa) with glass (infinite stiffness) used as a reference. Cells are plated on cover slips coated with PAA gels (of different crosslinking grade) as well as with Laminin and Poly-D-Lysine as described previously (Moshayedi et al., 2010).
Aging is a multifactorial process at the synaptic site, with alterations in protein expression levels, receptor abundances and deficits in synaptic potentiation. Astrocytes appear to play a pivotal role in these processes, as indicated by observed changes their supply of signaling molecules and cholesterol, as well as altered expression of proteins involved in neuron-glia communication (Perea et al., Mauch et al). Moreover, astrocytic secretion of ECM molecules critically contributes to the mechanoelastic properties of circuits in the brain and its change contributes to the increase rigidity of the extracellular environment increases in the aging brain. Such changes in of rigidity or viscoelasticity is sensed by neuronal mechanoreceptors and directly impacts on neuronal morphology and gene expression (Franze, 2013). However, the molecular mechanism of astrocytic aging and their effect on the ECM are poorly understood. To address the consequences of observed age-dependent changes in viscoelasticity, we recently adopted polyacrylamide (PAA)-based hydrogels to mimic these age-dependent changes in stiffness from young (soft, compliant, 0.1 kPa) to adult (1 kPa) and to very stiff (10 kPa) with glass (infinite stiffness) used as a reference. Cells are plated on cover slips coated with PAA gels (of different crosslinking grade) as well as with Laminin and Poly-D-Lysine as described previously (Moshayedi et al., 2010).
Hypothesis: Alterations in astrocytic protein homeostasis lead to impaired neuron-glia signalling and contribute to dysfunctional synaptic signalling in aging via altering mechanosensitive properties. Rejuvenation of the multipartite synapse may be achieved by combinatorial pharmacological targeting of astrocytic and neuronal key components that regulate protein homeostasis and neuron-astroglial communication. Our Aims are:
- To genetically target the expression of astrocytic proteins mediating of aging and analyse the effects on neuronal protein homeostasis as well as the multipartite synapse.
- To rejuvenate synaptic function by pharmacologic targeting key aging factors of astrocytic origin and identify putative synergistic effects with protein homeostasis in both astrocytes and neurons.
- To decipher age-dependent alterations in neuronal and astrocytic viscoelasticity and intervene with these through treatment with spermidine and drugs affecting mechanosensitive cation channels.
Astrocytic and mechanosensitive factors contribute to aging-related synaptic plasticity.
Collaborations: TP2 Stork (mechanisms of autophagy induction) TP11 Leßmann (characterization of synaptic function), TP3/4 Kreutz (mechanosensitive modulation of synaptic aging). TP6 Dityatev and TP8 Gundelfinger/Seidenbecher (contribution of the ECM to viscoelasticity modulation).