TP4: Molecular underpinnings of high-risk aging: Neuronal insulin signaling, amyloidosis and the metabolic syndrome (Michael R. Kreutz)
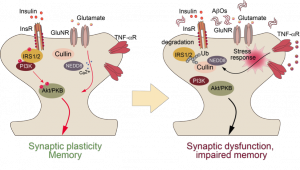
 The metabolic syndrome (MetS) has become a major threat for healthy living and active aging in western societies. It is characterized by overweight, high blood pressure, disturbed lipid metabolism, and insulin resistance. Largely ignored is the cognitive decline induced by the MetS that is correlated with age, inflammatory status, and amyloid load (Guillemot-Legris & Muccioli, 2017). Several studies suggest that both, insulin resistance and pro-inflammatory signaling, are risk factors for synaptic function. Of note, amyloid load and synaptic insulin resistance increase the risk for AD by a factor of 7-10. In recently published work we could show that posttranslational modifications impact the mechanism by which Amyloid-β induces synaptic dysfunction (Grochowska et al., 2017): the prominent isoform, pyroglutamylated Aβ3(pE)-42, induces synaptic dysfunction to a similar extent like Aβ1-42, but by clearly different mechanisms. In contrast to Aβ1-42, Aβ3(pE)-42 does not directly associate with synaptic membranes, but is instead taken up by astrocytes and, thus, potently induces glial release of the pro-inflammatory cytokine TNFα. Collectively, these data point to a scenario where neuroinflammatory processes together with direct synaptotoxic effects are caused by posttranslational modification of soluble oligomeric Aβ and contribute synergistically to the onset of synaptic dysfunction in AD.
The metabolic syndrome (MetS) has become a major threat for healthy living and active aging in western societies. It is characterized by overweight, high blood pressure, disturbed lipid metabolism, and insulin resistance. Largely ignored is the cognitive decline induced by the MetS that is correlated with age, inflammatory status, and amyloid load (Guillemot-Legris & Muccioli, 2017). Several studies suggest that both, insulin resistance and pro-inflammatory signaling, are risk factors for synaptic function. Of note, amyloid load and synaptic insulin resistance increase the risk for AD by a factor of 7-10. In recently published work we could show that posttranslational modifications impact the mechanism by which Amyloid-β induces synaptic dysfunction (Grochowska et al., 2017): the prominent isoform, pyroglutamylated Aβ3(pE)-42, induces synaptic dysfunction to a similar extent like Aβ1-42, but by clearly different mechanisms. In contrast to Aβ1-42, Aβ3(pE)-42 does not directly associate with synaptic membranes, but is instead taken up by astrocytes and, thus, potently induces glial release of the pro-inflammatory cytokine TNFα. Collectively, these data point to a scenario where neuroinflammatory processes together with direct synaptotoxic effects are caused by posttranslational modification of soluble oligomeric Aβ and contribute synergistically to the onset of synaptic dysfunction in AD.
Hypothesis: Based on preliminary work we hypothesize that Cullins are the upstream E3-ubiquitin ligases involved in IRS-1 and -2 degradation and that neddylation of Cullins sets the threshold for insulin resistance and in turn promotes amyloidosis and cellular senescence. We set therefore the following Aims:
- To provide an inventory of the neddylated synaptic proteome in synaptic aging and determine neddylation pathways in the synapse. We will develop technologies to specifically target neddylation at synapses.
- To probe intervention with neddylation for a rejuvenation of synapses. To this end we will also study the association of NEDD8 activating enzyme (NAE) with synaptic junctions.
A neuroinflammatory signal induced by amyloidosis promotes synaptic insulin resistance by degradation of the Insulin Receptor Substrate.
Collaborations: TP1/9 Dieterich (metabolic labeling and aging mechanisms in in vitro), TP12 Düzel (MetS and training in human subjects), TP2/10 Stork (behavioral phenotyping, synaptic and interneuron markers). TP6 Dityatev (in vivo electrophysiology). With our external partner Robert Ahrends (ISAS, Dortmund) we will conduct MS experiments.